SUMMARY. Hydrogen is one element receiving significant attention. As an energy source, hydrogen has long been considered a possible sustainable energy in the future. But it cannot be viewed in isolation, since it is both in competition and interdependent with other energies, and the technologies that use them. The question is whether hydrogen can be an important energy carrier of the future. Shell has been involved in hydrogen production as well as in hydrogen research, development and application for decades, with a dedicated business unit, Shell Hydrogen. In cooperation with the German research institute and think-tank Wuppertal Institute, Shell has conducted a study on hydrogen as a future energy carrier. The Shell Hydrogen Study looks at the current state of hydrogen supply pathways and application technologies and explores the potential and prospects for hydrogen as an energy source for the transportation sector. Currently, however, the aviation and maritime sectors have the lowest technology readiness levels in applying hydrogen energy.
THE ELEMENT HYDROGEN. Hydrogen was the first element created after the Big Bang. It is the most common substance in the universe and the richest energy source for stars like the sun. Hydrogen (H) is the first element in the periodic table of chemistry and is also the smallest, lightest atom. Pure hydrogen occurs on Earth only in molecular form (H2). Hydrogen on Earth is usually found in compounds, most notably as water molecules (H2O). First discovered in the 18th century, hydrogen was originally known as “inflammable air.” In the 19th century, hydrogen was featuring in contemporary visions of the future, especially in relation to the energy industry and locomotion. In the 1960s and 1970s, space travel and the increasing scarcity of resources further intensified the aura of excitement surrounding hydrogen. Since the 1990s the interest in hydrogen has been boosted by the growing urgency to find sustainable energy sources. More recently, the focus has been on hydrogen’s role in an increasingly electricity-based energy economy. Owing to its special physical properties, hydrogen is an almost permanent gas, since it only liquefies at very low temperatures (below –253°C). It has a low density, so it is usually stored under pressure. Liquefaction increases its density by a factor of 800. The most characteristic property of hydrogen is its flammability. It also has the highest gravimetric energy density (how much energy it contains in comparison to its mass) of all energy sources in use today. Due to its chemical properties, hydrogen has to be handled with care.
SUPPLY PATHWAYS. Since hydrogen usually exists on Earth as part of a compound, it has to be synthesized in specific processes in order to be used as a material or energy source. This can be achieved by different technical methods, and various primary energy sources both fossil and renewable fuels, in solid, liquid or gaseous form, and can be used in these technical production processes. The most important primary energy source for hydrogen production currently is natural gas at 70%, followed by oil, coal and electricity (as a secondary energy resource). Steam reforming (from natural gas) is the most commonly used method for hydrogen production. Other production methods include partial oxidation, auto-thermal reforming and gasification, which generally use fossil primary energy sources. Some unused residual hydrogen is available for energy use as a by-product of industrial production processes.
To date, only small amounts of hydrogen have been generated from renewable energies, although that amount is set to increase in the future. Electrolysis currently accounts for around 5% of global hydrogen production, but most of this is still based on conventional electricity sources. Electrolysis from surplus renewable power is seen as offering huge potential for the future. Alkaline electrolysis has been used in the industry for more than a century. Alternative electrolysis methods offering improved performance parameters (regarding conversion efficiency, flexibility and cost) are currently in development. Hydrogen production from biomass, while technically feasible, is still insignificant on a global scale, and while thermochemical methods such as biomass gasification and biogas reforming are already in use, biochemical processes are still in their infancy. Biomass has to be checked against sustainability, since it is a limited resource.
As the main hydrogen supply pathways, steam reforming of natural gas and biogas and electrolysis have been analyzed and compared in terms of energy input, Greenhouse Gas (GHG) emissions and production costs: Electrolysis based on conventional electricity (grid mix) requires high primary energy input. By contrast, natural gas and biogas reforming and electrolysis based on renewable electricity require little primary energy. Moreover, electrolysis of renewable electricity uses only minimal amounts of fossil resources. H2 originating from electrolysis with electricity from renewables produces the lowest GHG emissions, whereas H2 obtained from gas reforming – natural gas or biogas is better than hydrogen from grid-based electrolysis.
Of all the production methods considered, centralized hydrogen production is more cost-effective than production in smaller, decentralized plants. Centralized natural gas reforming is most cost-effective of all, with production costs of 1 to 2 EUR per kg of hydrogen. Electrolysis is significantly more expensive, and its commercial viability largely depends on electricity prices. The costs of biomass-based hydrogen production are between natural gas reforming and electrolysis. In the future, decentralized natural gas reforming, centralized electrolysis and centralized biomass routes are expected to offer the greatest cost-saving potential.
STORAGE & TRANSPORTATION. The specific physical and chemical properties of hydrogen lead to higher logistics costs in storage and transportation than other energy carriers. Hydrogen has a very low volumetric energy density, thus it has to be compressed for storage and transportation. Most important is the hydrogen storage as compressed gas. For end users, high-pressure storage tanks of varying design (350, 700 bar) are available.
A higher density for storage can be achieved by liquefaction, although this involves cooling the hydrogen to –253°C. The higher the storage density, the greater the amount of energy needed for cooling and compression, which is why more efficient storage methods are being explored. Unlike electricity, hydrogen can be successfully stored in large amounts for extended periods. Low-pressure underground storage facilities such as caverns can be filled with hydrogen from surplus renewable electricity and used as buffer stores for the electricity sector. As yet, there are very few underground hydrogen storage facilities in use. Novel storage media are materials-based hydrogen storage technologies.
At present, hydrogen is generally transported by lorry in pressurized gas tanks, and in some cases also in cryogenic liquid tanks. However, a lorry trailer can only carry so much gaseous hydrogen or liquid hydrogen. Regional hydrogen pipelines are available in some locations, the longest being in the USA and Western Europe. In the long-term, the natural gas supply infrastructure (pipelines and underground storage facilities) could also be used for the storage and transportation of hydrogen. In terms of transport costs, liquid hydrogen is suitable for long-distance transport; compressed gaseous hydrogen is suitable for shorter distances in smaller amounts; while pipelines are advantageous for large volumes.
MARITIME APPLICATION. Hydrogen is a highly versatile basic chemical with two main areas of use: material applications and energy applications. The most important material applications in industry are ammonia synthesis, primarily used for the production of nitrogenous fertilizers, and methanol synthesis. Also, hydrogen is a by-product of crude oil refining in refineries, in particular catalytic reforming of naphthas, on the other hand it is used for the processing and refining oil products in refineries in hydrotreating and hydrocracking. Energy applications involve converting the energy in hydrogen into heat, power or electricity.
In the shipping industry, diesel engines are used almost exclusively today. Ocean-going vessels use either heavy fuel oil or marine diesel as fuel, while inland waterway vessels within the EU use commercial diesel fuel. To date the only relevant alternative drive option for the shipping industry is the use of liquefied natural gas (LNG) or compressed natural gas (CNG) to fuel ships. As in aviation, fuel cells are currently being tested as energy providers for the on-board power supply. The functional capability of fuel cell modules has been tested successfully under maritime conditions (e4ships 2016). Fuel cells work more efficiently than comparable diesel-generator sets, in the partial load range in particular and through the possibility of combined heat and power generation. Air pollutants and noise emissions in ports can be reduced. In many cases the fuel cells are operated not with hydrogen but with other fuels, including methanol, natural gas or diesel fuel. These offer the advantages of greater availability, lower price and easier storage. They are converted into hydrogen with the aid of internal or external reformers.
The use of hydrogen-powered fuel cells for ship propulsion, by contrast, is still at an early design or trial phase – with applications in smaller passenger ships, ferries or recreational craft. The low- and high-temperature fuel cell (PEMFC) and the solid oxide fuel cell (SOFC) are seen as the most promising fuel cell types for nautical applications (EMSA 2017). As yet, however, no fuel cells have been scaled for and used on large merchant vessels.
In comparison to the efficient, slow-running diesel engine, which runs on heavy fuel oil, the power train and fuel are far too expensive. International technical standards need to be developed to use gaseous fuels such as hydrogen (Würsig/Marquardt 2016).
Submarines are a niche application of fuel cells. For instance, electrolysers have been used in submarines for some time now to produce oxygen for breathing air. Submarines operating with fuel cells have been developed in the USA and Germany. The submarines developed in Germany use PEM fuel cells and metal hydride hydrogen stores. In terms of submarine applications, fuel cells are characterized by low noise emissions, low operating temperatures and air-independent operation. However, the market for submarines is quite small, and even in the future it will not grow beyond a niche size.
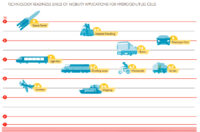
MOBILITY APPLICATIONS. Hydrogen can be used as an energy source for mobility applications. Initially, it was also tested in internal combustion engines, but in the transport sector hydrogen is now used almost exclusively in fuel cells. Space travel provided the historical and technical impetus for the development of hydrogen and fuel cell technology. In principle, hydrogen fuel cell systems are suitable for virtually all means of transport, but their technological maturity varies according to the means of transport and the way in which it is used. The technological maturity of a product can be determined in terms of Technology Readiness Levels (TRL), a system developed by the US space authority NASA. The TRL scale has levels 1-9. Sufficient technological maturity, which means at least proven functionality in the field of use at TRL 8, is a crucial prerequisite for a market launch in the respective mobility application area. At TRL 5-6, there are no plans as yet for commercial aircraft or merchant ships, but fuel cells can be used as an efficient energy source for auxiliary power units (APUs).
This article describes the promise of Hydrogen, and summarizes the maritime application of hydrogen. To read its application to other transport sectors, download the full report:
https://hydrogeneurope.eu/sites/default/files/shell-h2-study-new.pdf